M184V/I Prevalence
Emerging High-Level Resistance May Impact Future Treatment Options
An analysis of resistance data revealed that

M184V/I can predict significant resistance to the backbone component of many currently prescribed STRs2
High-level resistance appeared in all ARV drug classes among treatment-experienced patients1
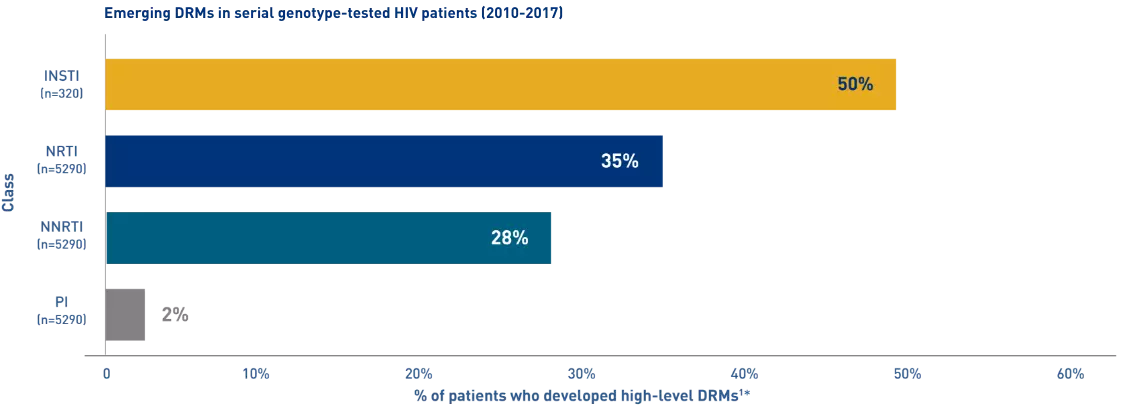
*The presence of drug resistance mutations may not correlate with clinical outcomes.
ARV=antiretroviral; DRM=drug resistance mutation; INSTI=integrase strand transfer inhibitor; NNRTI=non-nucleoside reverse transcriptase inhibitor; NRTI=nucleoside reverse transcriptase inhibitor; PI=protease inhibitor; STR=single tablet regimen.
References: 1. Kagan RM, Dunn KJ, Snell GP, et al. Trends in HIV-1 drug resistance mutations from a U.S. reference laboratory from 2006 to 2017. AIDS Res Hum Retroviruses. 2019;35(8):698-709. 2. Department of Health and Human Services. Guidelines for the use of antiretroviral agents in adults and adolescents with HIV. Updated January 20, 2022. Accessed March 8, 2022. https://clinicalinfo.hiv.gov/en/guidelines/adult-and-adolescent-arv/whats-new-guidelines
