The Only STR Recommended by the DHHS Guidelines for RI with Phase 3 Data1
Study design
DIAMOND: Phase 3, open-label, single-arm, prospective, multicenter study (DIAMOND was not a registrational trial. AMBER was the registrational trial which studied treatment-naïve patients).2,3


Key endpoints were:
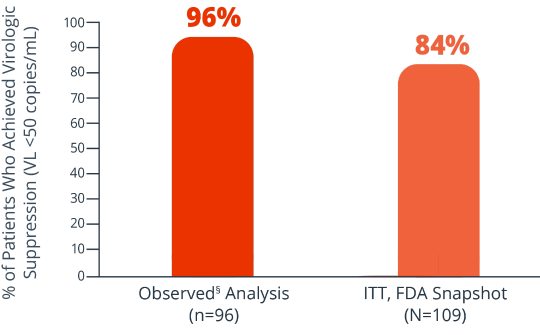
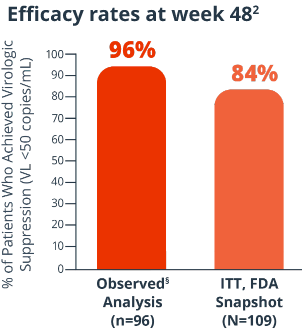
Proportion of patients with VL <50 copies/mL at 48 weeks (ITT, FDA Snapshot); proportion of patients with VL <50 copies/mL or <200 copies/mL at 48 weeks (observed analysis).2§
Key endpoints were: Proportion of patients with VL <50 copies/mL at 48 weeks (ITT, FDA Snapshot);
proportion of patients with VL <50 copies/mL or <200 copies/mL at 48 weeks (observed analysis).2§
Prior to or when initiating SYMTUZA®, patients should undergo testing for HBV and renal function. Appropriate labs, including liver testing, hepatitis serology, and HIV genotypic resistance testing, should be conducted and patients should be monitored during treatment as clinically appropriate.5
Limitations:
Single-arm, open-label, noncomparator design: study participants were motivated to start ART and had access to transportation, no-cost ART, and other trial-related services. Factors that may limit generalizability include differences in treatment implementation by study site, the inability to quantify those unwilling to participate, small proportion of women, and certain AIDS-related conditions were excluded.2
Limitations: Single-arm, open-label, noncomparator design: study participants were motivated to start ART and had access to transportation, no-cost ART, and other trial-related services. Factors that may limit generalizability include differences in treatment implementation by study site, the inability to quantify those unwilling to participate, small proportion of women, and certain AIDS-related conditions were excluded.2
Efficacy rates at week 482
8% of patients had virologic failure (≥50 copies/mL) and 7% had no VL data (ITT, FDA Snapshot)2
- No patients had protocol-defined virologic failure, and there were no study discontinuations due to lack of efficacy
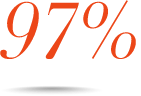
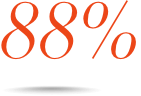
Consistent efficacy across multiple subpopulations, including MSM, Black, Hispanic, and 18 to 25 years old6¶
Patients who achieved VL <50 copies/mL at Week 48 in the observed§ analysis#:
MSM
(n=74)
Hispanic
(n=44)
Black/African American
(n=30)
18 to 25 years old
(n=32)
Patients who achieved VL <50 copies/mL at Week 48 (ITT, FDA Snapshot analysis)#:
MSM
(n=82)
Hispanic
(n=48)
Black/African American
(n=35)
Black/African American
(n=35)
Hispanic
(n=48)
18 to 25 years old
(n=38)

treatment-emergent mutations in rapid initiation2
*Evaluations could be done sooner based on availability of results.3
†Screening/baseline safety laboratory findings were evaluated with the following stopping criteria (retesting of abnormal screening/baseline safety laboratory values was allowed once): eGFR (MDRD formula) <50 mL/min, AST or ALT ≥2.5 times the ULN, serum lipase ≥1.5 times the ULN, positive pregnancy test for women of childbearing potential, laboratory results that the investigator believes should result in discontinuation of study medication, or active hepatitis C infection that, in the opinion of the investigator, required immediate treatment or was expected to require treatment during the study with agents not compatible with the components of SYMTUZA®.3
‡Resistance was evaluated based on predicted genotypic sensitivity (there was no exclusion based on the presence of specific RAMs). Patients who did not show full genotypic sensitivity to the components of SYMTUZA® (assessed using GenoSure Prime®) were stopped; an exception was resistance to lamivudine/emtricitabine associated with the M184I/V mutation alone.2
§Observed=patients with missing data were excluded from the analysis.2
¶Observed analysis: 90% of women (n=10) achieved VL <50 copies/mL at Week 48.6
#This is a post hoc subgroup analysis that is not powered for safety or efficacy; statistical significance has not been established.6
ALT=alanine aminotransferase; ART=antiretroviral therapy; ARV=antiretroviral; AST=aspartate aminotransferase; DHHS=Department of Health and Human Services; eGFR=estimated glomerular filtration rate; FTC=emtricitabine; HBV=hepatitis B virus; ITT=intent-to-treat; MDRD=Modification of Diet in Renal Disease; MSM=men who have sex with men; PrEP=pre-exposure prophylaxis; RAM=resistance-associated mutation; RI=rapid initiation; STR=single-tablet regimen; TDF=tenofovir disoproxil fumarate; ULN=upper limit of normal; VL=viral load.
References: 1. Department of Health and Human Services. Guidelines for the use of antiretroviral agents in adults and adolescents with HIV. Updated January 20, 2022. Accessed March 8, 2022. https://clinicalinfo.hiv.gov/en/guidelines/adult-and-adolescent-arv/whats-new-guidelines 2. Huhn GD, Crofoot G, Ramgopal M, et al. Darunavir/cobicistat/emtricitabine/tenofovir alafenamide in a rapid-initiation model of care for human immunodeficiency virus type 1 infection: primary analysis of the DIAMOND study. Clin Infect Dis. 2020;71(12):3110-3117. 3. Eron JJ, Orkin C, Gallant J, et al; AMBER Study Group. A week-48 randomized phase-3 trial of darunavir/cobicistat/emtricitabine/tenofovir alafenamide in treatment-naive HIV-1 patients. AIDS. 2018;32(11):1431-1442. 4. Supplementary to: Huhn GD, Crofoot G, Ramgopal M, et al. Darunavir/cobicistat/emtricitabine/tenofovir alafenamide in a rapid-initiation model of care for human immunodeficiency virus type 1 infection: primary analysis of the DIAMOND study. Clin Infect Dis. 2020;71(12):3110-3117. 5. SYMTUZA® [package insert]. Titusville, NJ: Janssen Therapeutics, Division of Janssen Products, LP. 6. Anderson D, Bolan R, DeJesus E, et al. Rapid initiation of darunavir/cobicistat/emtricitabine/tenofovir alafenamide (D/C/F/TAF) in patients with human immunodeficiency virus (HIV)-1 infection: age, race/ethnicity, and gender subgroup analyses from the DIAMOND Study. Poster presented at: 17th European AIDS Conference; November 6-9, 2019; Basel, Switzerland.