In Virologically Suppressed Patients,Viral Suppression Was Maintained at Week 48 and 96
Study design
EMERALD: Phase 3, randomized, open-label, international, multicenter, noninferiority study that randomized 1141 treatment-experienced adults to SYMTUZA® (n=763) vs continuing on bPI + FTC/TDF (n=378). Patients had been on therapy for ≥6 months with no history of virologic failure on darunavir-based regimens and were virologically suppressed prior to and at screening. After Week 48, patients could continue on or switch to SYMTUZA® in an open-label extension phase until Week 96.*
Key endpoints: Proportion of patients with virologic rebound at Week 48 (noninferiority margin 4%); proportion of patients with VL <50 copies/mL at 48 weeks (FDA Snapshot).1,2
Virologic response rates (FDA Snapshot)1,2
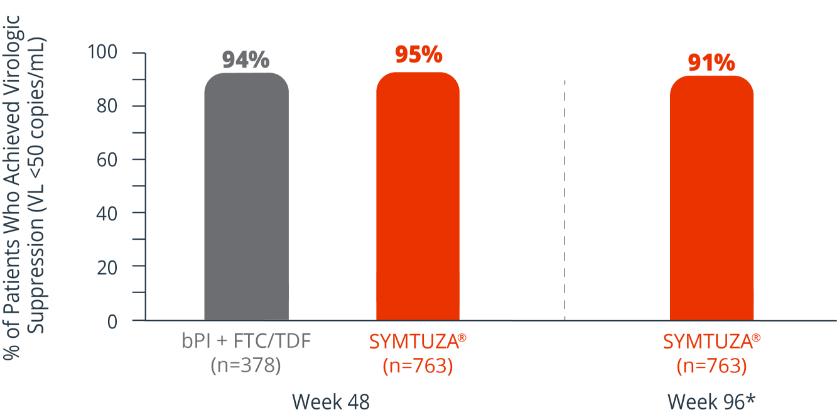
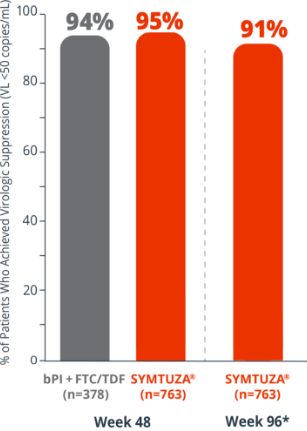

- 1% virologic failure rate (≥50 copies/mL) in the SYMTUZA® arm vs 1% in the bPI + FTC/TDF arm at 48 weeks1,2
- 1% virologic failure rate in the SYMTUZA® arm at 96 weeks2*
- 4% of patients in the SYMTUZA® arm had no virologic data at 48 weeks vs 6% in the bPI + FTC/TDF arm1
- 8% of patients in the SYMTUZA® arm had no virologic data at 96 weeks2*
of patients across both treatment arms with archived emtricitabine RAMs (n=53), mainly at reverse transcriptase position M184, maintained virologic suppression at Week 48 or at latest time point assessed3
*Week 96 was an open-label, single-arm extension, not a primary endpoint.2
bPI=boosted protease inhibitor; FTC=emtricitabine; RAM=resistance-associated mutation; TDF=tenofovir disoproxil fumarate; VL=viral load.
References: 1. Orkin C, Molina JM, Negredo E, et al; EMERALD Study Group. Efficacy and safety of switching from boosted protease inhibitors plus emtricitabine and tenofovir disoproxil fumarate regimens to single-tablet darunavir, cobicistat, emtricitabine, and tenofovir alafenamide at 48 weeks in adults with virologically suppressed HIV-1 (EMERALD): a phase 3, randomised, non-inferiority trial. Lancet HIV. 2018;5(1):e23-e34. 2. Eron JJ, Orkin C, Cunningham D, et al; EMERALD Study Group. Week 96 efficacy and safety results of the phase 3, randomized EMERALD trial to evaluate switching from boosted-protease inhibitors plus emtricitabine/tenofovir disoproxil fumarate regimens to the once daily, single-tablet regimen of darunavir/cobicistat/emtricitabine/tenofovir alafenamide (D/C/F/TAF) in treatment-experienced, virologically-suppressed adults living with HIV-1. Antiviral Res. 2019;170:104543. 3. SYMTUZA® [package insert]. Titusville, NJ: Janssen Therapeutics, Division of Janssen Products, LP.

